Peter,
I saw your numbers regarding the percentage of SO2 in K-meta and Na-meta and it seemed low compared to what I have always read. I looked back to a reference I had and this is the info posted. Not saying you are incorrect but trying to understand the numbers.
source of info:
http://www.brsquared.org/wine/Articles/SO2/SO2.htm
<CENTER>
<H2>3. Sodium and Potassium Salts </H2></CENTER>Two salt forms of sulphite are generally used in winemaking: potassium metabisulphite (K<SUB>2</SUB>S<SUB>2</SUB>O<SUB>5</SUB>) and sodium metabisulphite (Na<SUB>2</SUB>S<SUB>2</SUB>O<SUB>5</SUB>).
The molecular weight of sodium metabisulphite is 190.2 and that of potassium metabisulphite is 222.4, whereas that of sulphur dioxide (SO<SUB>2</SUB>) is 64.1. The salts dissociate giving two moles of SO<SUB>2</SUB> for each mole of the salt. Thus, the SO<SUB>2</SUB> content of sodium metabisulphite is 2 x 64.1/190.2 = 67.4% and that of potassium metabisulphite is 2 x 64.1/222.4 = 57.6%.
<CENTER>
Table 1. SO<SUB>2</SUB> content in metabisulphite salts
<TABLE border=1>
<T>
<TR>
<TH align=middle>Salt</TH>
<TH>SO<SUB>2</SUB> content</TH></TR>
<TR>
<TD>Sodium metabisulphite</TD>
<TD align=middle>67.4 %</TD></TR>
<TR>
<TD>Potassium metabisulphite</TD>
<TD align=middle>57.6 %</TD>
<TD></TD></TR></T></TABLE></CENTER>
Winemakers generally prefer to use the potassium form for sulphite additions, since this increases the level of potassium in the wine which may later help to precipitate tartrates when cold stabilising. Others claim that the sodium form can contribute a `salty' flavour to wine.
<CENTER>
<H2>4. Forms and Functions of Sulphur Dioxide in Wine </H2></CENTER>
<H3>4.1. Dissociation of Forms</H3>Potassium metabisulphite dissociates in water to potassium ions (K<SUP>+</SUP>) and singly ionised bisulphite, (HSO<SUB>3</SUB>)<SUP>-</SUP>. (Sodium metabisulphite dissociates in the same way.)
Metabisulphite dissociates in the following way to form these fractions:
<CENTER>K<SUB>2</SUB>S<SUB>2</SUB>O<SUB>5</SUB> + H<SUB>2</SUB>O ===> 2K<SUP>+</SUP> + 2(HSO<SUB>3</SUB>)<SUP>-</SUP>
</CENTER>
Sulphur dioxide is a bifunctional acid, and dissociates into three fractions. The quantity of each of these fractions depends on the thermodynamic constants and the pH. The dissociation is almost instantaneous.
The three fractions are molecular SO<SUB>2</SUB> (SO<SUB>2</SUB>), sulphite (SO<SUB>3</SUB><SUP>2-</SUP>), and bisulphite (HSO<SUB>3</SUB><SUP>-</SUP>). Dissociation of the various fractions is almost immediate.
Since wine is acidic, hydrogen ions are present (H<SUP>+</SUP>) and the bisulphite (HSO<SUB>3</SUB><SUP>-</SUP>) can then transform into sulphur dioxide:
<CENTER>
<TABLE>
<T>
<TR>
<TD>HSO<SUB>3</SUB><SUP>-</SUP>
<TD>+ H<SUP>+</SUP></TD></TD>
<TD><===> </TD>
<TD>H<SUB>2</SUB>O</TD>
<TD>+ SO<SUB>2</SUB></TD></TR>
<TR>
<TD>singly ionized bisulphite </TD>
<TD>+ hydrogen ion</TD>
<TD></TD>
<TD>water</TD>
<TD>+ unionized (molecular) sulphur dioxide </TD></TR></CENTER></T></TABLE>
Additionally,
<CENTER>
<TABLE>
<T>
<TR>
<TD>HSO<SUB>3</SUB><SUP>-</SUP> </TD>
<TD>+ H<SUB>2</SUB>O </TD>
<TD><===> </TD>
<TD>H<SUP>+</SUP> </TD>
<TD>+ SO<SUB>3</SUB><SUP>2-</SUP> </TD></TR>
<TR>
<TD>singly ionized bisulphite </TD>
<TD>+ water</TD>
<TD></TD>
<TD>hydrogen ion</TD>
<TD>+ doubly ionized sulphite </TD></TR></T></TABLE>
</CENTER>
Thus, the relationships of the forms of SO<SUB>2</SUB> in wine are shown completely by:
<CENTER>
<TABLE>
<T>
<TR>
<TD>H<SUB>2</SUB>O + </TD>
<TD>SO<SUB>2</SUB> </TD>
<TD><===> </TD>
<TD>H<SUP>+</SUP> </TD>
<TD>+ (HSO<SUB>3</SUB>)<SUP>-</SUP> </TD>
<TD><===> </TD>
<TD>2H<SUP>+</SUP> </TD>
<TD>+ SO<SUB>3</SUB><SUP>2-</SUP> </TD></TR>
<TR>
<TD>water + </TD>
<TD>molecular sulphur dioxide </TD>
<TD></TD>
<TD>hydrogen ion </TD>
<TD>+ bisulphite </TD>
<TD></TD>
<TD>hydrogen ion </TD>
<TD>+ sulphite </SUP></TD></TR></T></TABLE></CENTER>
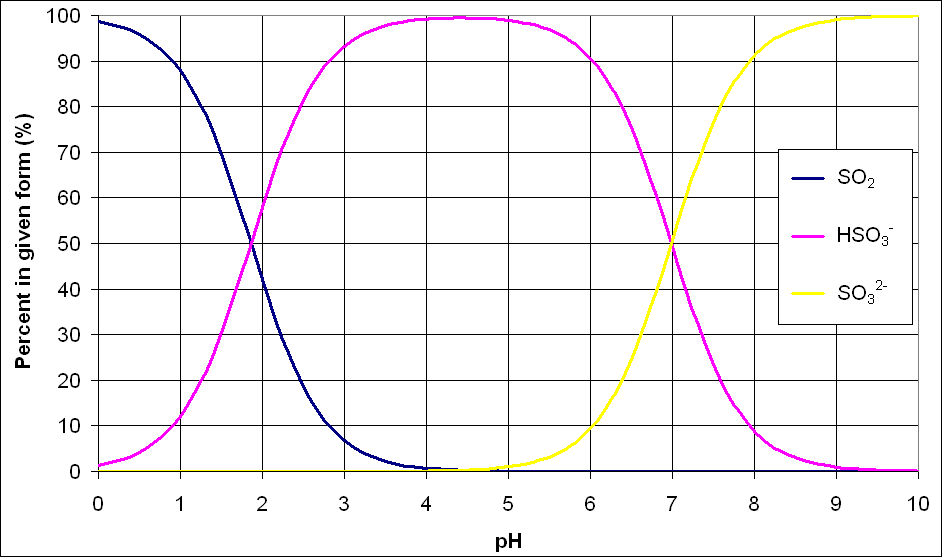
The amount of each free SO<SUB>2</SUB> that is in each fraction (bisulphite, sulphite, and molecular) is determined by the pH. Figure 1 shows the distribution of the different species for various pH values.
<CENTER>
</CENTER>






 amespace prefix = o ns = "urn
amespace prefix = o ns = "urn chemas-microsoft-com
chemas-microsoft-com