seth8530
The Atomic Wine Maker
Ok guys, I need your help with something.
First off most of us know the equation
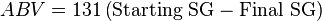 to give a pretty good approximation of the your ABV is. However, since we do not know where the 131 in this equation comes from I will refer us to a different equation used by brewers.
to give a pretty good approximation of the your ABV is. However, since we do not know where the 131 in this equation comes from I will refer us to a different equation used by brewers.
This equation uses a little bit of chemistry to justify where the where the constant comes from in front of the equation.

"Where 1.05 is the number of grams of ethanol produced for every gram of CO2 produced, and .79 is the density of ethanol"
The guaranteed problem with the latter equation is that it does not take into account the fact that your FG does not accurately reflect the sugar content of your your wine/beer/mead because the alcohol is throwing off the density of your must. I somehow doubt that the first equation takes this into account as well because any factor needed to correct this problem would need to be attached to the final gravity term.
So, I am proposing a revised set of equations. That go to the form of this
ABV=131*(SG-FG*K)
Or the other Above equation where FG is always multiplied by a factor K.
The purpose and objective of "K" is to make your FG equal to what it would be if that FG was obtained by a pure water sugar mix. Pretty much, to get rid of the skewing caused by the alcohol. To do this I will need some data to find the proportionality constant "K" that relates the specific gravity of a mixture with just sugar and water in it to a mixture that has sugar water and a known amount of alcohol in it.
So the question is.. How do we find K?
For that I will need some help from the community. I need people who have accurate metric scales that can get down to the gram in accuracy and people who have measuring equipment that is accurate to the ml. As well as accurate thermometers to make gravity corrections for temperature. These people will also need to have some dry 40% liqiour on hand and some table sugar.
I believe that this correction factor "K" is proportional to the alcohol concentration in the wine/mead/beer.
And to obtain data I need at least three volunteers for consistencies sake. The experiment should not take more than a few hours but before I start writing up an experimental procedure I would really like to see what kind of internist there is in coming up with this correction factor.
So, if interested in helping me perform this experiment please give me a shout out!
First off most of us know the equation
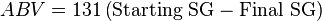
This equation uses a little bit of chemistry to justify where the where the constant comes from in front of the equation.

"Where 1.05 is the number of grams of ethanol produced for every gram of CO2 produced, and .79 is the density of ethanol"
The guaranteed problem with the latter equation is that it does not take into account the fact that your FG does not accurately reflect the sugar content of your your wine/beer/mead because the alcohol is throwing off the density of your must. I somehow doubt that the first equation takes this into account as well because any factor needed to correct this problem would need to be attached to the final gravity term.
So, I am proposing a revised set of equations. That go to the form of this
ABV=131*(SG-FG*K)
Or the other Above equation where FG is always multiplied by a factor K.
The purpose and objective of "K" is to make your FG equal to what it would be if that FG was obtained by a pure water sugar mix. Pretty much, to get rid of the skewing caused by the alcohol. To do this I will need some data to find the proportionality constant "K" that relates the specific gravity of a mixture with just sugar and water in it to a mixture that has sugar water and a known amount of alcohol in it.
So the question is.. How do we find K?
For that I will need some help from the community. I need people who have accurate metric scales that can get down to the gram in accuracy and people who have measuring equipment that is accurate to the ml. As well as accurate thermometers to make gravity corrections for temperature. These people will also need to have some dry 40% liqiour on hand and some table sugar.
I believe that this correction factor "K" is proportional to the alcohol concentration in the wine/mead/beer.
And to obtain data I need at least three volunteers for consistencies sake. The experiment should not take more than a few hours but before I start writing up an experimental procedure I would really like to see what kind of internist there is in coming up with this correction factor.
So, if interested in helping me perform this experiment please give me a shout out!
Last edited:




