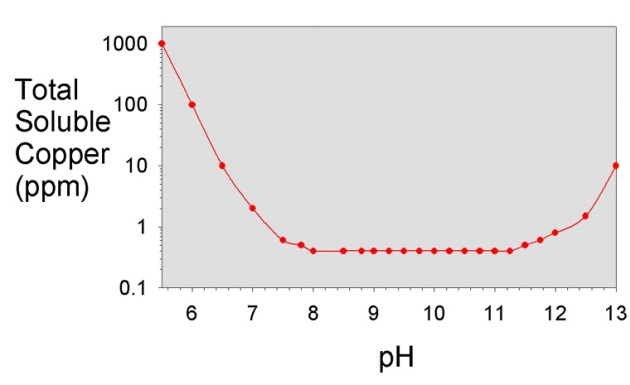
I was looking at the "how to" information on a wine making supply web site and came across the wine making troubleshooting part of the site and read their recommended procedure for getting rid of sulfur smell in wine.
These steps are included in their recommended procedure
- Buy a piece of copper flashing from a home supply store
- Hold the piece of copper in the neck of the carboy while the wine is being racked, so that the wine runs over the copper surface and into the carboy. Fine and/or filter the wine.
This is counter to what I feel is the proper way to handle this issue, due to the potential excess copper remaining in the solution. I brought this to their attention, sent articles etc, but they are steadfast that this is safe and correct advice. The site has been very responsive and I don't want anything but to have a discussion on this topic by knowledgeable home and commercial winemakers. I will be sending the site the link as well.
If you agree or don't agree, please give a short explanation why.
These steps are included in their recommended procedure
- Buy a piece of copper flashing from a home supply store
- Hold the piece of copper in the neck of the carboy while the wine is being racked, so that the wine runs over the copper surface and into the carboy. Fine and/or filter the wine.
This is counter to what I feel is the proper way to handle this issue, due to the potential excess copper remaining in the solution. I brought this to their attention, sent articles etc, but they are steadfast that this is safe and correct advice. The site has been very responsive and I don't want anything but to have a discussion on this topic by knowledgeable home and commercial winemakers. I will be sending the site the link as well.
If you agree or don't agree, please give a short explanation why.